HOUSTON, TX / ACCESSWIRE / March 21, 2023 / EON Smarter Body Contouring, created by Dominion Aesthetic Technologies, Inc., ["Dominion"] has received FDA Clearance for the back and thighs. This is the third FDA Clearance for EON, the first robotic, touchless, 1064nm laser used for non-invasive lipolysis. EON was previously FDA-cleared for clinical providers to treat patients' full abdomen and flanks.
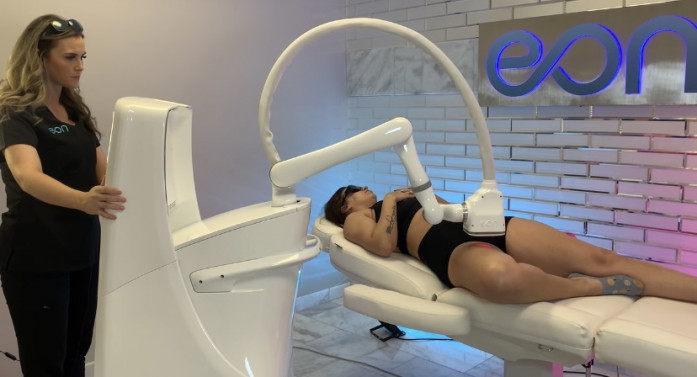
EON Inner Thigh Treatment
"Receiving Clearance from the FDA for these new indications for EON is a great benefit for our patients. I'm delighted our research report was accepted, again validating our technology. I would especially like to recognize the key efforts of Dominion's research team in Winter Park (Florida), who were essential for making this happen," stated Thomas Fiala, MD, MBA, FACS, FRCSC.
EON uses a time-proven 1064 nm laser that heats fat up to 123.8F, causing fat-cell death with natural flushing through the lymphatic system. Proprietary jet-impingement cooling and real-time monitoring create a comfortable patient experience. During treatment, the robotic device hovers over the patient's body, memorizing the topography and using sensors to detect skin temperature, distance from the skin, laser energy, jet temperature, and room temperature. These features are part of EON's advanced safety protocol, which includes over forty connected sensors. One treatment consists of multiple segments, and a segment runs for twenty minutes. Flanks can be treated in forty minutes, and the entire abdomen can be treated in sixty minutes. EON has unrivaled efficacy, and is free of time-consuming gels, applicators, and post-treatment massages which are often needed with other body contouring devices.
"With our new FDA Clearance, EON is proving, while using state-of-the-art technology, that we can adapt to any environment to benefit all patients and offer providers maximum flexibility. We will continue to improve our device and its uses to ensure that EON keeps its place as the premium device for body contouring," said Cooper Collins, Dominion Chief Executive Officer.
EON is available through a network of medical providers across the United States. For more information on EON or to find a provider near you, please visit www.eonlaser.com
DOMINION AESTHETIC TECHNOLOGIES, Inc. ["Dominion"]
Founded in 2016, Dominion is a laser platform aesthetic device company with corporate headquarters in Texas and R&D labs in Florida. Dominion aims to offer the best solutions for aesthetic physicians and practitioners by merging the expertise of its renowned Scientific Advisory Committee with its respected Research & Development team. Dominion created EON - Smarter Body Contouring to exceed the unmet needs of aesthetic physicians by leveraging innovative touchless robotic technology to advance the aesthetic body contouring industry. Please visit www.dominionaesthetic.com or explore more about EON by visiting www.eonlaser.com.
Contact Information
Sarah Ramsey
Director of Marketing and Comm - Dominion Aesthetic Technologies
sramsey@dominionaesthetic.com
Related Files
SOURCE: Dominion Aesthetic Technologies, Inc.
View source version on accesswire.com:
https://www.accesswire.com/744914/EON-the-First-Robotic-Body-Contouring-Device-Gains-Additional-FDA-Clearance-for-Back-and-Thighs
